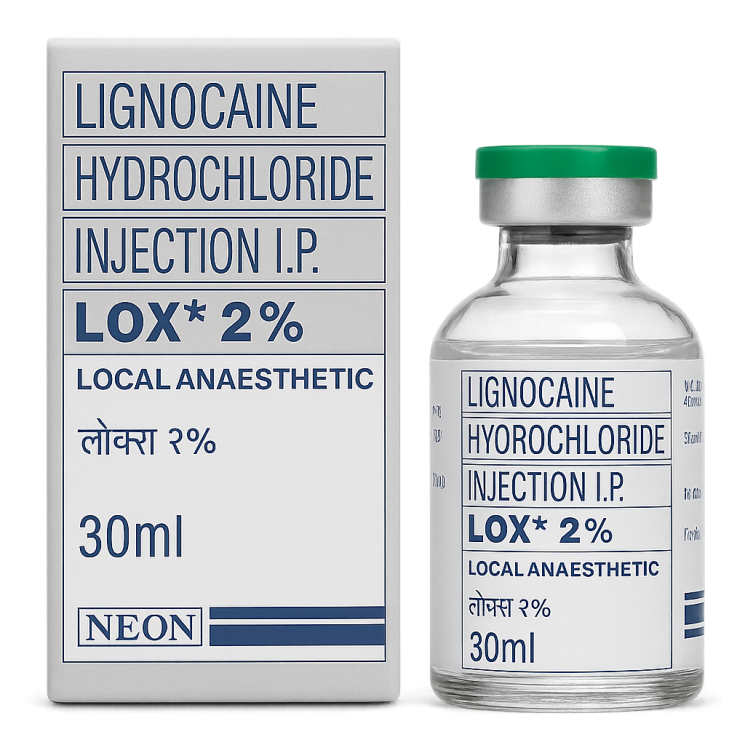
Lignocaine Hydrochloride Injection 2% (NEON)
CONSULTATION WITH A SPECIALIST
It is not blocked for now — DOWNLOAD IMO
Place an order via messengers:
Lignocaine Hydrochloride Injection 2% (NEON)
Product Name: Лидокаина гидрохлорид инъекции, Lignocaine Hydrochloride Injection, Lidocainhydrochlorid Injektion, Inyección de clorhidrato de lidocaína, Injection de chlorhydrate de lidocaïne, حقن هيدروكلوريد الليدوكائين, การฉีดลิโดเคนไฮโดรคลอไรด์, Лидокаин гидрохлорид инъекцияси, Лидокаин гидрохлорид инъекциясы, Lidokain hidroklorid inyeksiya, Инъекция гидрохлорид лидокаин, Лидокаин гидрохлорид инъекція, לִידוֹקאין הידרוכלוריד להזרקה
Main Indications for Lignocaine Hydrochloride Injection 2%: Infiltration anesthesia, nerve block anesthesia, epidural anesthesia, spinal anesthesia, local anesthesia for minor surgical procedures, local anesthesia in dentistry, management of ventricular arrhythmias.
Lidocaine hydrochloride is a local amide-type anesthetic. It blocks voltage-gated sodium channels in nerve membranes, preventing the conduction of pain impulses and causing a reversible loss of sensation at the injection site. The drug is characterized by a rapid onset of action — usually from one to five minutes — and a medium duration of effect from sixty to one hundred and twenty minutes, depending on the method of administration and tissue vascularization. In cardiology, it is used as an antiarrhythmic agent for ventricular rhythm disorders, especially in the context of acute myocardial infarction or cardiac glycoside intoxication.
In field conditions, with a complete lack of medical care, Lignocaine Hydrochloride can be used for pain relief in trauma, suturing, minor surgical manipulations, and dental interventions, as well as a means to manage life-threatening ventricular arrhythmias. For infiltration anesthesia, a 0.5–2% solution is used, administered in small doses of one to two milliliters in a fan-like pattern around the injured area. The maximum total dose for such administration should not exceed 300 mg of the active substance, which corresponds to 15 ml of a 2% solution for an adult weighing about 70 kg. For nerve block anesthesia, 2–5 ml of the 2% solution is injected near a nerve trunk, avoiding blood vessels, which provides loss of sensation for one to two hours. To manage ventricular arrhythmias in the absence of other means, the drug is administered intravenously slowly at a dose of approximately 1 mg per kilogram of body weight, which is 3.5–5 ml of a 2% solution for an average-built adult.
Dosage for Lignocaine Hydrochloride Injection 2%
It is very important not to exceed the maximum permissible dose of 5 mg per kilogram of body weight. This means that for a weight of 50 kg, the allowable limit is about 250 mg, or 12 ml of a 2% solution; for a weight of 60 kg, the limit is 300 mg, or 15 ml of solution; for a weight of 70 kg — 350 mg, or approximately 17 ml of solution; for a weight of 80 kg — 400 mg, or about 20 ml of solution; for a weight of 90 kg — 450 mg, which corresponds to approximately 22 ml of solution. Before administration, it is necessary to ensure that the needle is not in a blood vessel by gently pulling back the syringe plunger and confirming the absence of blood. The solution is administered slowly, monitoring the patient's condition. If numbness of the lips, dizziness, difficulty breathing, or other signs of intoxication appear, administration should be stopped immediately. The dosage form of the drug is a sterile solution for injection 2% (20 mg/ml) in 30 ml vials. Excipients include sodium chloride to maintain osmolarity and methylparaben as a preservative, which allows for multiple withdrawals of the solution from one vial and prevents bacterial contamination. The drug should be stored at a temperature below 30 °C, avoiding freezing; in an opened vial, the solution can be used for one week, provided sterility is maintained and there is no cloudiness. Contraindications include hypersensitivity to amide anesthetics, pronounced bradycardia, severe atrioventricular block, and decompensated heart failure. Possible side effects include dizziness, drowsiness, tremor, convulsions, arterial hypotension, bradycardia, rhythm disturbances, and allergic reactions.
Toxicity and Biosafety — Lignocaine Hydrochloride Injection 2%
Lidocaine hydrochloride belongs to the amide-type local anesthetics with moderate systemic toxicity. According to experimental studies, acute toxicity for intravenous administration in mice is LD₅₀ ≈ 22 mg/kg body weight, for subcutaneous administration LD₅₀ is in the range of 300–400 mg/kg. In rats, LD₅₀ for oral administration is estimated to be within 600–700 mg/kg. For dogs, toxic effects are observed at an intravenous dose of about 10–15 mg/kg. These values indicate a relatively narrow therapeutic window for systemic administration.
The main toxic manifestations of overdose include neurotoxic effects (excitation, convulsions, depression of the respiratory center) and cardiotoxic effects (arterial hypotension, bradycardia, ventricular arrhythmias, asystole). The cumulative toxicity of the drug is formed by the action of the active substance lidocaine; excipient components, including sodium chloride, are pharmacologically neutral at therapeutic concentrations. Methylparaben, used as a preservative, has extremely low acute toxicity (LD₅₀ for rats for oral administration is about 2–3 g/kg body weight), and its contribution to the overall toxicity of the solution is minimal.
Thus, the main toxic load of the drug is associated with the action of lidocaine. When modeling the cumulative toxicity based on the content of 20 mg/ml of the active substance in the vial, the critical dose for an adult weighing 70 kg occurs with the administration of approximately 350–400 mg (17–20 ml of solution). This corresponds to the maximum safe dose used in clinical practice. The biosafety of the drug is considered satisfactory provided that dosages and administration rules are strictly followed.
Synergy — Lignocaine Hydrochloride Injection 2%
Lidocaine hydrochloride, being a representative of amide local anesthetics, demonstrates significant pharmacological synergy when combined with various agents and substances used in biomedical practice. It has been established that the combination of lidocaine with adrenaline has a potentiating effect: vasoconstriction reduces the rate of systemic absorption of the anesthetic, which leads to an extension of the anesthesia time and a reduction in the risk of systemic toxicity. This synergy is characterized as additive in terms of duration of action and protective in terms of systemic effects. Under conditions of tissue ischemia, the effect can be modulating, as a higher concentration of the active substance is maintained at the injection site. Interaction with opioid agonists (e.g., morphine or fentanyl) manifests as enhanced analgesia in regional pain management methods, which is explained by different sites of action: opioids act primarily on opioid receptors, while lidocaine blocks sodium channels. This mechanism allows for a pronounced potentiating effect, reducing the required dose of each agent and minimizing the risk of side effects.
The combination of lidocaine with non-steroidal anti-inflammatory drugs has an additive nature, enhancing both local and systemic analgesic effects through the combined influence on pain and inflammation mediators (prostaglandins, sodium channels). When used concomitantly with magnesium sulfate, a modulating interaction is noted: magnesium ions block NMDA receptors and potentiate the analgesic effect of lidocaine, while simultaneously reducing hyperalgesia. Concurrent use with ketamine demonstrates pronounced synergy due to additional inhibition of NMDA receptors, providing a protective effect against neuroplastic changes in the nociceptive system.
An important direction of lidocaine synergy is associated with its interaction with antioxidants and membrane protectors, including α-tocopherol and glutathione. Experimental data indicate that such combinations have a protective effect on cells during ischemic damage, reducing the risk of oxidative stress induced by the administration of high doses of the anesthetic. Interaction of lidocaine with corticosteroids during regional administration has also been noted: corticosteroids have anti-inflammatory and membrane-stabilizing effects, which further enhances the local effectiveness of anesthesia.
Thus, lidocaine hydrochloride demonstrates a wide range of confirmed synergies: potentiation of duration of action when combined with adrenaline, enhancement of analgesia when combined with opioids, a modulating effect in combination with magnesium sulfate and ketamine, additive interaction with NSAIDs, and a protective effect in combination with antioxidants and membrane stabilizers. These effects have tissue-specific directionality (nervous and muscle tissue), a systemic nature (analgesia, cardiomodulation), and a cellular level of implementation (inhibition of sodium channels, blockade of NMDA receptors, reduction in the production of inflammatory mediators).
References: PubMed ID: 28479200; PubMed ID: 30738922; ScienceDirect DOI: 10.1016/j.pain.2012.04.017; SpringerLink DOI: 10.1007/s00540-020-02806-5.
Pharmacodynamics of Lignocaine Hydrochloride Injection 2%
The pharmacodynamic profile of lidocaine is determined by its ability to reversibly block voltage-gated sodium channels in nerve cell membranes, which prevents the occurrence and propagation of action potentials along nerve fibers. This effect is most pronounced in small unmyelinated C-fibers and thin myelinated Aδ fibers, which explains the predominant suppression of pain and temperature sensitivity while preserving deep proprioceptive function at low concentrations. With increasing concentration, blockade of larger nerve fibers, including motor fibers, occurs. Thus, the effect of lidocaine is realized on a gradient from local analgesia to complete anesthesia.
Lidocaine acts not only on peripheral nerves but also has systemic effects. In the cardiac system, it acts as a class Ib antiarrhythmic agent, reducing the automaticity of ectopic foci and shortening the action potential of cardiomyocytes by blocking sodium channels during the depolarization phase. This leads to suppression of ventricular ectopic activity, reduction in the frequency of ventricular tachyarrhythmias, and improvement of myocardial electrical stability.
In the central nervous system, lidocaine has a dose-dependent effect. In therapeutic doses with systemic administration, it can reduce neuronal excitability, modulate nociceptive cascades, and exhibit a mild sedative effect. At higher concentrations, exceeding the safe threshold, a neurotoxic effect occurs: excitation, paresthesia, tremor, and convulsions, which is associated with diffuse blockade of sodium channels in the cerebral cortex.
Lidocaine also exhibits anti-inflammatory properties, as confirmed by its influence on the production of pro-inflammatory cytokines and inhibition of neutrophil migration. In some models, antioxidant and membrane-stabilizing effects have been noted, which are associated with limiting the formation of reactive oxygen species and stabilizing cell membranes. These effects are realized at the cellular level and may be significant in tissue damage accompanied by inflammation and ischemia.
Thus, the pharmacodynamics of lidocaine includes local action on nerve fibers, systemic antiarrhythmic effects on the myocardium, modulation of pain cascades at the level of the central nervous system, as well as additional anti-inflammatory and membrane-protective action. The effect of the drug can be classified as tissue-specific (nervous tissue, myocardium), systemic (analgesia, cardiomodulation), and cellular (blockade of ion channels, inhibition of inflammatory mediators).
References: PubMed ID: 23222913; PubMed ID: 29597235; Wiley DOI: 10.1111/j.1476-5381.2012.01971.x; SpringerLink DOI: 10.1007/s40122-020-00155-y.
Pharmacokinetics of Lignocaine Hydrochloride Injection 2%
With parenteral administration, lidocaine hydrochloride is rapidly absorbed from the injection site; the rate of absorption depends on tissue vascularization and the presence of vasoconstrictive agents. With intravenous administration, absorption does not limit the action, as the active substance immediately enters the systemic circulation. After entering the circulation, the substance actively binds to plasma proteins, mainly albumin and α1-acid glycoprotein, which determines the distribution dynamics and the possibility of displacement by other compounds. Distribution occurs predominantly in well-perfused organs — the heart, liver, kidneys, lungs, and brain. In muscle tissue and fat depots, the substance is partially deposited and serves as a reservoir with subsequent slow release into the blood.
Metabolism occurs mainly in the liver with the participation of microsomal enzymes of the cytochrome P450 system. The main pathways of biotransformation include N-dealkylation and hydroxylation, resulting in the formation of pharmacologically active and inactive metabolites. The isoenzymes CYP3A4 and CYP1A2 play an important role in the metabolism, the activity of which can change under the influence of concomitant substances. Metabolites undergo further conjugation and are excreted primarily in the urine. A small amount is excreted in the bile.
With intramuscular administration, delayed entry into the bloodstream is observed, which prolongs the duration of action compared to intravenous administration. With local and regional application, a significant part of the dose remains in the tissues, providing a local effect, and then is gradually absorbed. With transdermal or mucosal administration (gels, sprays), absorption depends on the thickness and condition of the epithelial barrier. In this case, a limited amount of the active substance enters the systemic circulation, reducing the likelihood of systemic toxicity.
Excretion occurs primarily through the kidneys, in the form of metabolites and to a lesser extent unchanged. Secretion through the renal tubules plays an important role, so impaired renal function may slow down elimination. Accumulation in tissues is minimal with therapeutic use; however, with repeated administration of high doses, an accumulation effect in adipose tissue and myocardium is possible.
References: PubMed ID: 32227929; PubMed ID: 23222913; ScienceDirect DOI: 10.1016/S0140-6736(71)91371-4; SpringerLink DOI: 10.1007/s00228-008-0476-5.
Mechanisms of Action and Scientific Rationale: Lignocaine Hydrochloride Injection 2%
The effect on the liver and gastrointestinal tract is primarily associated with metabolism in hepatocytes. Lidocaine actively undergoes N-dealkylation under the action of cytochrome P450 isoenzymes, which determines its biotransformation and detoxification. An additive interaction with the liver enzyme systems, including CYP3A4 and CYP1A2, is observed. Metabolites have less pharmacological action but participate in modulating antioxidant activity, reducing the production of reactive oxygen species, and stabilizing membrane structures. This gives the drug membrane-stabilizing and partially antioxidant properties at the cellular level.
Reference: PubMed ID: 32227929
The immune system is influenced by lidocaine's ability to reduce neutrophil migration and inhibit the production of pro-inflammatory cytokines such as TNF-α and IL-1β. This effect can be characterized as modulating and anti-inflammatory, realized at the tissue and cellular levels. The influence occurs through the inhibition of NF-κB and MAPK signaling cascades, which reduces the expression of inflammatory mediators and limits tissue damage.
Reference: PubMed ID: 28008198
The impact on the nervous system is the main direction of action. Lidocaine blocks voltage-gated sodium channels of neurons, preventing the generation and conduction of action potentials. This effect is tissue-specific for peripheral nerves; however, in systemic concentrations, a central modulating effect also appears. The reduction in the excitability of nociceptive cascades, suppression of hyperalgesia, and regulation of synaptic transmission in the spinal cord determine its potentiating analgesic effect. At the cellular level, the effect is realized through the blockade of Nav1.7 and Nav1.8 sodium channels, as well as interaction with NMDA receptor systems at high concentrations.
Reference: Wiley DOI: 10.1111/j.1476-5381.2012.01971.x
Endocrine and metabolic regulation is affected indirectly. Lidocaine can alter microcirculation and influence vascular tone, which can have an additive effect when combined with other substances regulating metabolism. In some models, a reduction in the production of stress-induced hormones and normalization of local tissue perfusion have been noted. At the cellular level, the drug exhibits protective properties by reducing calcium overload and preventing the activation of apoptosis cascades.
Reference: SpringerLink DOI: 10.1007/s00540-020-02806-5
Thus, the mechanisms of action of lidocaine hydrochloride include membrane-stabilizing and antioxidant effects in the liver, anti-inflammatory and modulating effects on the immune system, blockade of sodium channels and modulation of pain cascades in the nervous system, as well as indirect participation in the regulation of endocrine-metabolic processes. The nature of the interaction can be classified as additive in relation to enzyme systems, modulating in relation to the immune response, potentiating in nociceptive cascades, and protective under metabolic stress.
Reference: PubMed ID: 23222913
| Weight, gross | 78 g |
| Made by | Asiabiopharm Co Ltd |
| Country of origin | Thailand |


0 reviews for Lignocaine Hydrochloride Injection 2% (NEON)