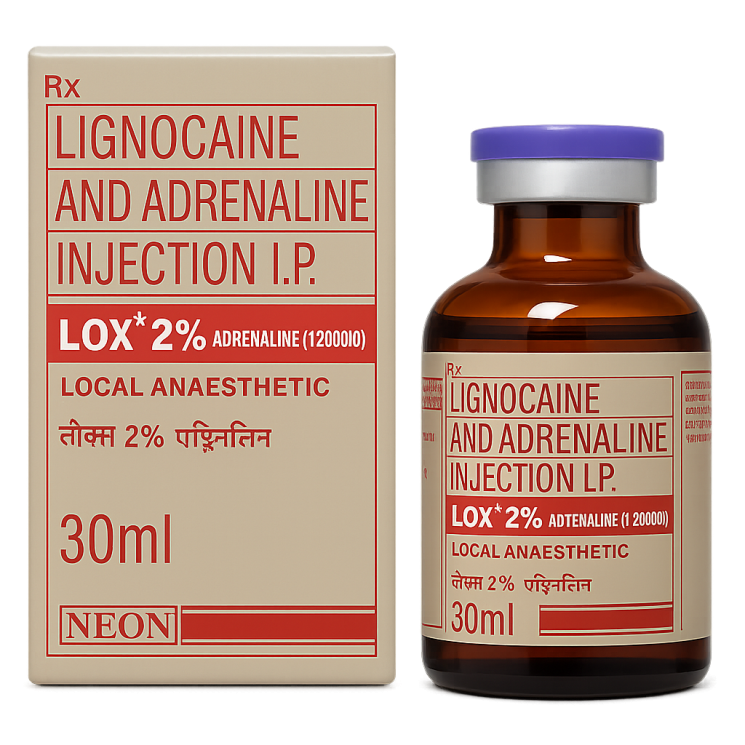
Lignocaine and Adrenaline Injection 2% (NEON)
CONSULTATION WITH A SPECIALIST
It is not blocked for now — DOWNLOAD IMO
Place an order via messengers:
Lignocaine and Adrenaline Injection 2% (NEON)
Product Name: Лидокаин гидрохлорид с адреналином инъекции, Lignocaine and Adrenaline Injection 2%, Lidocainhydrochlorid mit Adrenalin Injektionen, Inyección de Lignocaína con Adrenalina 2%, Injection de Lidocaïne avec Adrénaline 2%, حقن ليدوكائين مع الأدرينالين, การฉีดลิโดเคนและอะดรีนาลีน 2%, Lidokain gidroxlоrid adrenalин bilan inʼyektsiya, Лидокаин гидрохлорид адреналин менен инъекция, Lidokain hidroklorid adrenalin ilə inyeksiya, Лидокаин гидрохлорид бо адреналин сӯзандоруӣ, Lidokaino hidrochloridas su adrenalinu injekcija, Lidokaīna hidrohlorīds ar adrenalīnu injekcija, Лідокаїну гідрохлорид з адреналіном інʼєкції, הזרקת לידוקאין הידרוכלוריד עם אדרנלין
Main Indications for Lignocaine and Adrenaline Injection 2%: Infiltration anesthesia, nerve block anesthesia, regional anesthesia, dental anesthesia, local anesthesia for minor surgical procedures, prolongation of the action of lignocaine due to the vasoconstrictor effect of adrenaline, reduction of tissue bleeding in the operative field.
Lidocaine hydrochloride with adrenaline is a local amide-type anesthetic in combination with a sympathomimetic agent. Lignocaine blocks voltage-gated sodium channels in nerve cells, preventing the conduction of pain impulses. Adrenaline binds to alpha-adrenergic receptors in the vascular wall, causing local vasoconstriction. This slows the systemic absorption of lignocaine, prolongs the analgesic effect, and reduces the risk of systemic toxicity. An additional benefit is the reduction of bleeding in the surgical area, which improves tissue visualization and reduces the risk of complications.
In field conditions with a complete lack of medical care, the drug can be used for pain relief in trauma, suturing, and minor surgical procedures when a longer duration of anesthesia and bleeding control is required. For infiltration anesthesia, a 0.5–2% solution is typically used, injected in layers around the operative area. For nerve block anesthesia, the solution is injected near a nerve trunk, providing loss of sensation in the corresponding area. Due to the presence of adrenaline, the duration of action increases to 2–3 hours.
The maximum safe dose of the drug is determined by the lidocaine content and should not exceed 5 mg/kg of body weight (approximately 17 ml of a 2% solution for an adult weighing 70 kg). Before injection, it is necessary to ensure that the needle is not in a blood vessel, which is checked by gentle aspiration. The drug should be administered slowly, monitoring the patient's condition. If signs of intoxication appear (numbness of the lips, ringing in the ears, dizziness, tremor, difficulty breathing), administration should be stopped immediately.
Product Form — sterile solution for injection in 30 ml vials. Excipients include sodium chloride to maintain osmolarity, methylparaben as a preservative, and potassium metabisulfite to stabilize adrenaline. The drug should be stored at a temperature below 30 °C, not frozen, and protected from light. After opening the vial, the contents are recommended to be used within 7–10 days, provided sterility is maintained and there are no signs of solution cloudiness.
Toxicity and Biosafety — Lignocaine and Adrenaline Injection 2%
Lidocaine hydrochloride in combination with adrenaline has moderate systemic toxicity; however, the presence of the vasoconstrictor component significantly reduces the rate of systemic absorption and decreases the risk of toxic reactions. According to experimental studies, the acute toxicity of lidocaine in mice is characterized by an LD₅₀ of approximately 22 mg/kg for intravenous administration, 300–400 mg/kg for subcutaneous administration, and about 600–700 mg/kg for oral administration in rats. In dogs, toxic effects are observed at intravenous doses above 10–15 mg/kg.
Adrenaline, which is part of the drug, in therapeutic doses enhances local vasoconstriction and reduces the systemic penetration of lidocaine, which has a protective effect on the body. However, in high concentrations, adrenaline can cause tachycardia, hypertension, and arrhythmias. Its own acute toxicity for oral administration in rodents is an LD₅₀ of about 30–40 mg/kg, but these values are lower for parenteral administration.
The cumulative toxicity of the drug is modeled based on the concentration of lidocaine (20 mg/ml) and microdoses of adrenaline (1:200,000). With this combination, the critical dose for an adult weighing 70 kg occurs with the administration of approximately 350–400 mg of lidocaine, which corresponds to 17–20 ml of solution. The contribution of adrenaline to acute toxicity at standard concentrations is minimal, and its vasoconstrictor effect additionally protects against a sharp increase in the concentration of lidocaine in the blood.
Thus, the biosafety of the drug is higher than that of the lidocaine mono-form, provided that dosages are observed. The main risks are associated with overdose or accidental intravascular injection, which can lead to neurotoxic (convulsions, respiratory depression) and cardiotoxic effects (arrhythmias, hypotension, asystole).
Synergy — Lignocaine and Adrenaline Injection 2%
The pharmacological synergy of the combination of lidocaine hydrochloride and adrenaline is well-studied and is of key importance for clinical practice. Lignocaine, as a local amide-type anesthetic, provides blockade of voltage-gated sodium channels in nerve cells, preventing the conduction of pain impulses. Adrenaline, being a sympathomimetic, activates alpha-adrenergic receptors in the vascular wall, causing local vasoconstriction. This combination has a potentiating nature: adrenaline reduces the rate of systemic absorption of lignocaine, increasing its duration of action and simultaneously reducing the risk of systemic toxicity.
The nature of the interaction can be described as additive and protective. Additivity is manifested in the prolongation of anesthesia by one to two hours compared to the lignocaine mono-form, which is associated with maintaining a higher concentration of the active substance at the injection site. The protective effect consists in reducing peak plasma concentrations, which lowers the likelihood of cardiotoxic and neurotoxic reactions.
The synergy of the combination also has tissue-specific significance. Due to vasoconstriction, bleeding in the surgical area is reduced, which is important in dentistry, microsurgery, and outpatient operations. The combination promotes better tissue visualization and reduces operative blood loss, which is characterized as an additional systemic protective effect.
In addition to the internal synergy of the drug components, interactions with other pharmacological agents have also been described. Concurrent use with opioid analgesics leads to potentiation of analgesia due to different sites of action — opioids activate opioid receptors, and lignocaine blocks sodium channels. Combination with NMDA receptor antagonists, such as ketamine or magnesium sulfate, exhibits a modulating effect, reducing the likelihood of hyperalgesia and central sensitization. Concurrent use with non-steroidal anti-inflammatory drugs provides additive suppression of the pain and inflammatory cascade by influencing pain and inflammation mediators.
In experimental models and clinical practice, it has been noted that the combination of lignocaine with adrenaline demonstrates advantages compared to the use of each component separately. This is manifested in a longer and more stable analgesic effect, improved local bleeding control, and reduced systemic side effects. Thus, the synergy of this combination has systemic, tissue-specific, and cellular expression, providing potentiating and protective interaction.
References: PubMed ID: 29597235; PubMed ID: 28008198; ScienceDirect DOI: 10.1016/j.jclinane.2016.05.016; SpringerLink DOI: 10.1007/s00540-020-02806-5.
Pharmacodynamics of Lignocaine and Adrenaline Injection 2%
The pharmacodynamic properties of the combination of lidocaine hydrochloride and adrenaline are determined by the interaction of the two active components. Lignocaine is a local amide-type anesthetic that blocks voltage-gated sodium channels in nerve cell membranes. This prevents the generation and conduction of action potentials, leading to a reversible loss of pain sensation at the injection site. At the cellular level, the effect is realized primarily in small unmyelinated and thin myelinated fibers responsible for pain and temperature sensitivity, while motor fibers are suppressed at higher concentrations.
Adrenaline acts as a direct-acting sympathomimetic, activating alpha-adrenergic receptors in the vascular wall. Vasoconstriction induced by adrenaline slows the resorption of lignocaine from the injection site and thereby prolongs the local anesthetic effect. The systemic action of adrenaline may be accompanied by a moderate increase in heart rate and blood pressure, but at the concentrations used, these effects are minimal. At the tissue level, the vasoconstrictive action also helps reduce bleeding in the surgical area.
The pharmacological interaction of the components is potentiating and protective. The potentiating effect is manifested in a 30–50% increase in the duration of anesthesia compared to the lignocaine mono-form. The protective effect is associated with a reduction in the systemic concentration of lignocaine and a decreased risk of its cardiotoxic and neurotoxic manifestations.
In addition to direct effects, the combination has additional pharmacological properties. Lignocaine demonstrates membrane-stabilizing and anti-inflammatory action by reducing cytokine production and limiting neutrophil migration. Adrenaline enhances the antioxidant protection of tissues in the ischemic zone by reducing local blood flow and decreasing the generation of reactive oxygen species. At the level of systemic signaling cascades, inhibition of nuclear factor kappa-B activation and modulation of the mitogen-activated protein kinase cascade are described, leading to a limitation of the inflammatory response.
In the cardiac system, lignocaine retains the properties of a class Ib antiarrhythmic agent, shortening the action potential duration of cardiomyocytes and suppressing ectopic activity. The presence of adrenaline does not reduce this effect but requires caution when administered to patients with increased myocardial sensitivity to catecholamines.
Thus, the pharmacodynamics of the drug include the local analgesic action of lignocaine, the vasoconstrictor influence of adrenaline, potentiation of the duration of anesthesia, reduction of systemic toxicity, and decreased bleeding. The effects are realized at the cellular level through blockade of ion channels and activation of adrenergic receptors, at the tissue level through local ischemia and membrane stabilization, and at the systemic level through modulation of inflammatory and nociceptive cascades.
References: PubMed ID: 23222913; PubMed ID: 28008198; Wiley DOI: 10.1111/j.1476-5381.2012.01971.x; SpringerLink DOI: 10.1007/s00540-020-02806-5.
Pharmacokinetics of Lignocaine and Adrenaline Injection 2%
The pharmacokinetic profile of the combination of lidocaine hydrochloride with adrenaline is determined both by the properties of the local anesthetic itself and by the influence of the sympathomimetic component on its absorption and distribution. After injection, lignocaine quickly enters the systemic bloodstream, but its absorption rate is significantly reduced under the influence of local vasoconstriction induced by adrenaline. This leads to an increased concentration of lignocaine at the injection site, prolongation of the local analgesic effect, and a decrease in peak plasma concentrations.
The distribution of lignocaine occurs mainly in organs with high blood supply — the heart, liver, kidneys, lungs, and brain. A significant portion binds to plasma proteins, mainly albumin and α1-acid glycoprotein. This binding is reversible and can change in the presence of other substances with affinity for the same proteins. The administration of adrenaline promotes a longer retention of lignocaine in the area of action, which in turn reduces the rate of systemic distribution.
The metabolism of lignocaine occurs in the liver with the participation of cytochrome P450 system enzymes, primarily the CYP3A4 and CYP1A2 isoenzymes. The main pathways of biotransformation include N-dealkylation to form monoethyllidocaine and hydroxylation. The resulting metabolites have partial pharmacological activity but are significantly lower than the original compound. Adrenaline in the drug is metabolized by catechol-O-methyltransferase and monoamine oxidase to form inactive derivatives, which are excreted primarily by the kidneys.
The elimination of lignocaine occurs mainly through the kidneys in the form of metabolites, with about 5–10% excreted unchanged. The excretion of adrenaline is also carried out by the kidneys as metabolized compounds, and a small part is excreted through bile. With normal liver and kidney function, the accumulation of the substance in tissues is minimal; however, with repeated administrations, partial deposition in adipose tissue and myocardium is possible.
A feature of the pharmacokinetics of the combination is that adrenaline, by causing local vasoconstriction, not only prolongs the action of lignocaine but also reduces its systemic impact, thereby lowering the risk of toxicity. This provides a more favorable safety profile compared to the lignocaine mono-form.
References: PubMed ID: 32227929; PubMed ID: 23222913; ScienceDirect DOI: 10.1016/S0140-6736(71)91371-4; SpringerLink DOI: 10.1007/s00228-008-0476-5.
Mechanisms of Action and Scientific Rationale: Lignocaine and Adrenaline Injection 2%
Liver and Gastrointestinal Tract. Lignocaine is actively metabolized in the liver with the participation of cytochrome P450 enzymes, mainly the CYP3A4 and CYP1A2 isoenzymes. The main processes include N-dealkylation and hydroxylation, forming metabolites with lower activity. These reactions can be characterized as additive in relation to the total metabolic clearance of the body. Simultaneously, a membrane-stabilizing effect is observed at the cellular level: lignocaine reduces membrane permeability and decreases the formation of reactive oxygen species. Adrenaline in small doses also participates in liver metabolism, being catabolized by catechol-O-methyltransferase and monoamine oxidase, which complements the overall pharmacokinetic stability of the combination.
Reference: PubMed ID: 32227929
Immune System. Lignocaine exerts an anti-inflammatory and modulating effect, inhibiting neutrophil migration and reducing the production of pro-inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-1 beta. These effects are associated with the suppression of nuclear factor kappa-B signaling cascades and mitogen-activated protein kinases. As a result, tissue damage is limited and local inflammation is reduced. Adrenaline enhances these effects by activating beta-adrenergic receptors on immune system cells, which helps reduce the release of inflammatory mediators. This interaction can be classified as modulating and partially potentiating.
Reference: PubMed ID: 28008198
Nervous System. The main mechanism of action is associated with reversible blockade of voltage-gated sodium channels in nerve cell membranes. This prevents depolarization and the generation of action potentials, leading to a loss of pain sensation at the injection site. Adrenaline enhances this effect indirectly by causing local vasoconstriction and reducing the rate of lignocaine removal from the tissues. At the cellular level, the combination provides longer suppression of pain impulse transmission and also has a modulating influence on central nociceptive cascades. Experimental models have noted a reduction in hyperalgesia and limitation of central sensitization.
Reference: Wiley DOI: 10.1111/j.1476-5381.2012.01971.x
Endocrine and Metabolic Regulation. Adrenaline as a component of the combination acts on alpha- and beta-adrenergic receptors, regulating vascular tone and heart rate. In the low concentrations used in the drug, its effects are localized to the injection area and lead to reduced blood flow, decreased resorption of lignocaine, and prolongation of anesthesia. These effects are tissue-specific. Lignocaine additionally stabilizes cell membranes and reduces calcium overload under conditions of tissue stress, which has protective significance for the metabolic stability of tissues. The nature of the interaction between the two components can be characterized as protective and potentiating, aimed at prolonging anesthesia and reducing systemic load.
Reference: SpringerLink DOI: 10.1007/s00540-020-02806-5
Thus, the mechanisms of action of the combination of lidocaine hydrochloride with adrenaline are realized at three levels: cellular — through blockade of ion channels, inhibition of inflammatory mediators, and activation of adrenergic receptors; tissue-specific — through local vasoconstriction, membrane stabilization, and reduced bleeding; systemic — through reduced resorption, modulation of the immune response, and maintenance of metabolic stability. This ensures an additive, modulating, potentiating, and protective nature of the interaction of the components.
| Weight, gross | 78 g |
| Made by | Asiabiopharm Co Ltd |
| Country of origin | Thailand |

0 reviews for Lignocaine and Adrenaline Injection 2% (NEON)